- Home
- The colon targeting efficacies of mesalazine medications and their impacts on the gut microbiome
- The colon targeting efficacies of mesalazine medications and their impacts on the gut microbiome
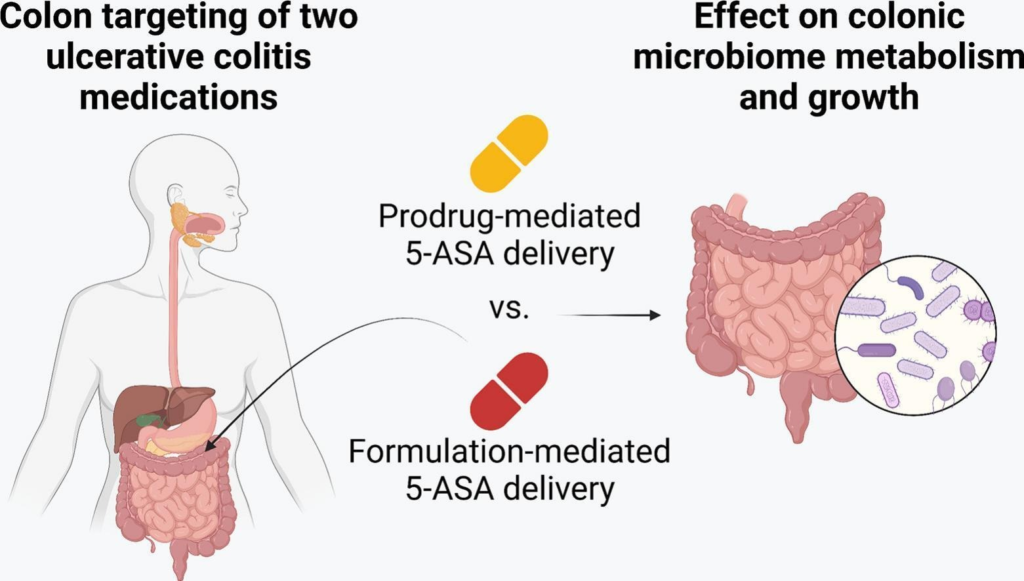
Treating ulcerative colitis (UC) requires more than selecting the right drug—it demands precise delivery to the site of inflammation. Mesalazine (5-ASA), a first-line anti-inflammatory agent, is commonly used in UC, but its effectiveness depends on how and where it's released in the gastrointestinal (GI) tract.
In a recent study, two 5-ASA delivery strategies were compared using the SHIME® in vitro GI model, which mimics both healthy and UC-affected gut conditions. The first strategy used sulfasalazine, a prodrug activated by colonic bacteria. The second used Octasa® (1600 mg), a pH- and bacteria-sensitive formulation designed to release 5-ASA in the lower GI tract.
Under healthy conditions, both delivered comparable 5-ASA levels to the colon. However, under UC-simulated conditions—where inflammation, pH, and microbiota are altered—Octasa® significantly outperformed sulfasalazine (P < 0.0001). This underscores the importance of disease-adapted drug delivery systems.
The study also assessed microbiome impacts. Both treatments reduced healthy gut bacteria and altered metabolite production (e.g., lactate, SCFAs like butyrate), with effects varying by gut condition.
The findings highlight the need for personalized, condition-specific 5-ASA delivery strategies to optimize treatment efficacy and minimize unintended microbiome disruption in UC patients.
Access the full article here.